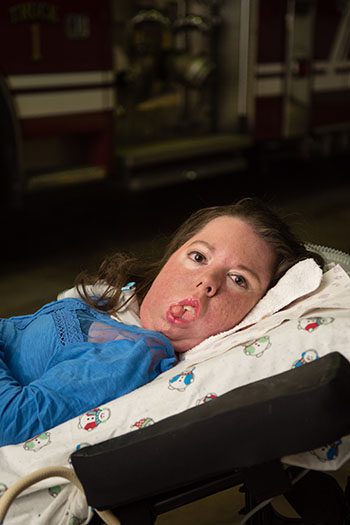
Meet the National Disability Employment Awareness Month (NDEAM) Panelists
October is National Disability Employment Awareness Month (NDEAM)! Hear directly from adults living with SMA in the workforce. Register for Cure SMA’s first NDEAM webinar. The 60-minute webinar will be held on Wednesday, October 24 at 2:00 p.m. CDT. Register today! Kaitlyn Yang @KaitlynYang At age nine, Kaitlyn Yang emigrated to the U.S. from China […]
Meet the National Disability Employment Awareness Month (NDEAM) Panelists Read More »