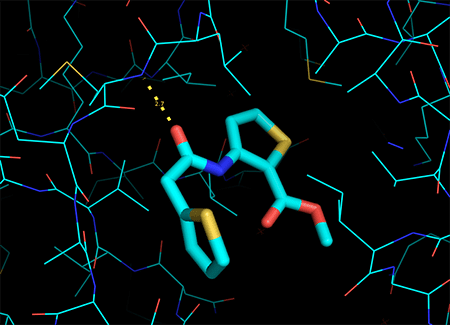
Update on the Clinical Development of LMI070
Novartis recently provided the following update on clinical trials for LMI070, an orally available SMA drug that corrects SMN2 splicing: “We have made the difficult decision to pause enrollment for our study of LMI070 for the treatment of Type 1 Spinal Muscular Atrophy (SMA). Animal safety studies were taking place in parallel with this trial, […]
Update on the Clinical Development of LMI070 Read More »