The recent availability of new treatments for SMA would not have been possible without the participation of SMA-affected individuals in clinical trials. Yet participating in clinical trials can be stressful. We at Cure SMA want to understand how future trials can be designed to reduce stress for participants and their parents and caregivers. To achieve these goals, we surveyed SMA clinical trial participants, and their parents and caregivers, about their experiences and attitudes. The results of this study were published in a manuscript titled, “The Cure SMA Clinical Trial Experience Survey: A Study of Trial Participant Perspectives on Clinical Trial Management and Patient-Centric Management Practices” in the peer-reviewed journal, Neurology and Therapy.1 We hope these insights will be help clinical trial sponsors design future SMA research protocols.
How We Did It
We used information from publications and discussions with parents and caregivers of SMA clinical trial participants to develop a survey about experiences with recruitment, enrollment, site visits, and interactions with study teams. We invited SMA clinical trial participants and their caregivers to complete the survey. Recruitment announcements were sent to these individuals through targeted email and were also included in Cure SMA’s research newsletter. The online survey was available from November 15, 2019, to January 10, 2020.
What We Found
For our analysis, we focused on seventy completed surveys that reflected the unique experiences individuals affected by SMA and their caregivers. Most respondents had positive experiences with many aspects of SMA clinical trials, including communication with the study team, screening and enrollment, trial logistics, and finding information about the trial. Respondents also found clinical research coordinators (CRCs) and principal investigators (PIs) understanding, trustworthy, and responsive. Time and logistical conflicts were the most frequently reported challenges.
We also asked respondents what motivated them to participate in a clinical trial. The most significant motivators were desire for clinical benefit and improved quality of life, access to an investigational drug, and the opportunity to help others with SMA. The top reported benefits of participation were hope for a better future, the opportunity to help others, positive relationships with the study team, and access to medical experts. (Table 11)
When asked which factors caused concern about trial participation, uncertainty about safety of the drug, uncertainty about whether potential benefits would justify potential risks, and concerns about potential adverse effects were weighted most heavily. The greatest reported sources of stress during the trials were concern about potential adverse events, fear of physical and/or mental pain that could accompany required tests, and challenges managing SMA-related medical complications.
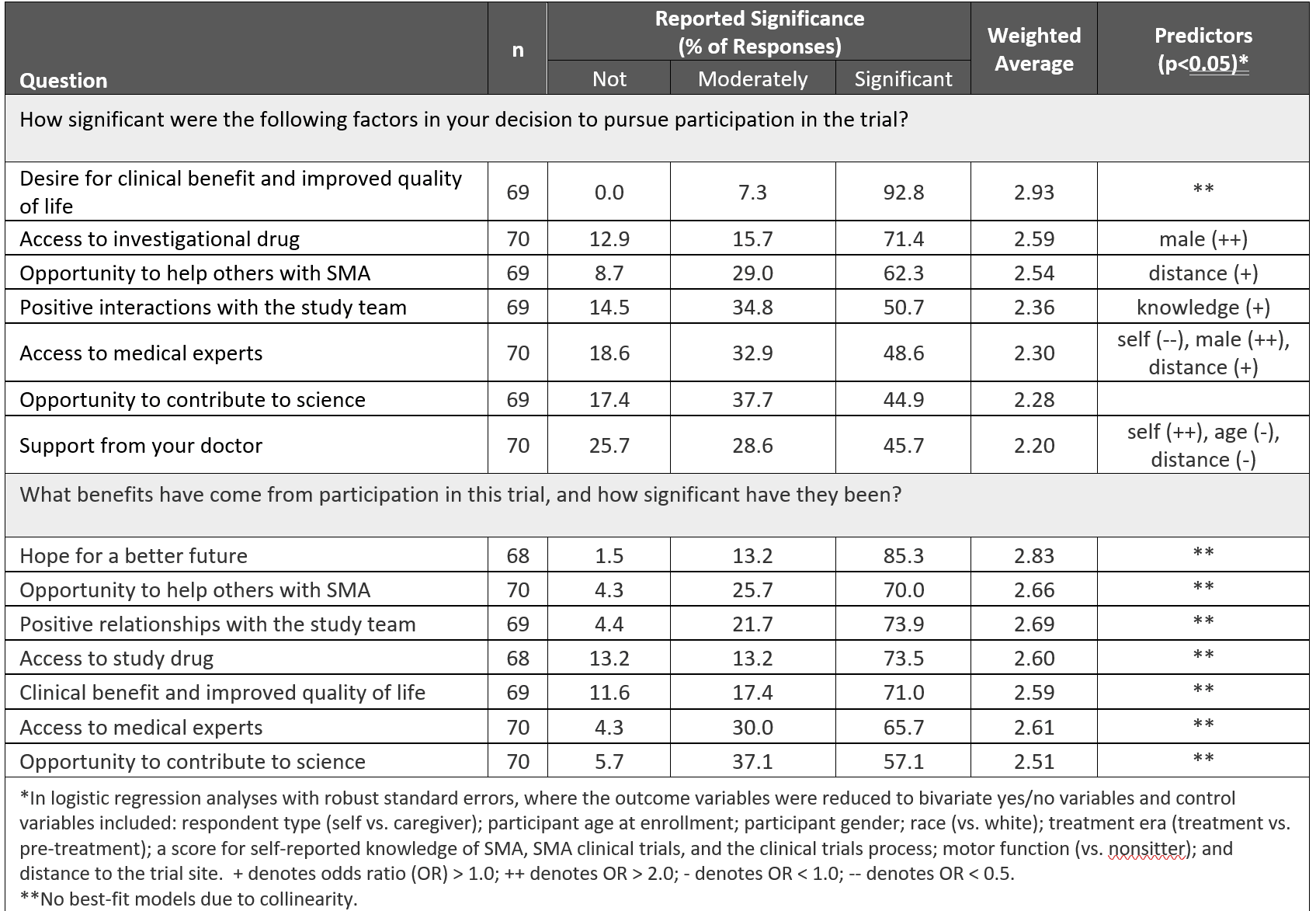
Table 1, “Motivators and benefits associated with SMA clinical trial participation,” by Petersen et al.1 has not been modified and is licensed under https://creativecommons.org/licenses/by-nc/4.0/.
Finally, we learned that understanding expressed by, trust in, and responsiveness of the study team were helpful in reducing stress during trials, and that participants thought clinical trial experiences could be improved by having access to more information, including information about the trial to and trial results.
Why It Matters
We hope that these research findings will be of interest to the SMA community and useful to sponsors and health care professionals involved in clinical trials. While we found that most survey respondents had a generally positive experience during SMA clinical trial participation, this research also shed light on opportunities to optimize trial design and conduct so that research participation is less stressful and more convenient for individuals with SMA and their caregivers. Those who would like to learn more can access our open access publication here.
Funding for this research was provided by members of the 2019 and 2020 Cure SMA Industry Collaboration, which included, Astellas, Biogen, Genentech/Roche, Novartis, Novartis Gene Therapies, Cytokinetics, and Scholar Rock.
About the Cure SMA Industry Collaboration

The Cure SMA Industry Collaboration (SMA-IC) was established in 2016 to leverage the experience, expertise, and resources of pharmaceutical and biotechnology companies, as well as other nonprofit organizations involved in the development of SMA therapeutics, to address a range of scientific, clinical, and regulatory challenges more effectively. It is currently comprised of our partners at Novartis Gene Therapies, Biogen, Genentech/Roche Pharmaceuticals, Scholar Rock, and SMA Europe.
References
- Peterson IS, Mazzella AJ, Belter LT, Curry MA, Cruz RE, Jarecki J. The Cure SMA Clinical Trial Experience Survey: A Study of Trial Participant Perspectives on Clinical Trial Management and Patient-Centric Management Practices. Neurol Ther. 2022.



