 Cure SMA is pleased to announce its participation in the 2021 Muscular Dystrophy Association (MDA) Virtual Clinical & Scientific Conference (March 15-18, 2021) and the American Academy of Neurology (AAN) 2021 Virtual Annual Meeting (April 17-22, 2021).
Cure SMA is pleased to announce its participation in the 2021 Muscular Dystrophy Association (MDA) Virtual Clinical & Scientific Conference (March 15-18, 2021) and the American Academy of Neurology (AAN) 2021 Virtual Annual Meeting (April 17-22, 2021).
Poster presentations at these meetings feature data from research initiatives launched by the Cure SMA Industry Collaboration (SMA-IC). The SMA-IC is a multi-faceted partnership that brings together pharmaceutical companies, Cure SMA, and other patient advocacy groups to share information, ideas, and data. The Collaboration works together to address scientific, clinical, and regulatory topics that are critical to advancing drug development in SMA and will benefit the broader SMA community partners. The ongoing goals of the SMA-IC include:
- Development and refinement of outcome measures.
- Distribution of adult and teen survey to evaluate unmet medical needs upon transition from pediatric to adult care.
- Distribution of a caregiver survey to assess quality of life within the caregiver community.
- Evaluation of opportunities to “bring trials to patients” via the adoption of telemedicine and remote monitoring.
- Development of a competency-based formal education program for SMA physical therapists.
Assessment of Current Best-Practices, Across Neurology and Cure SMA Care Centers in the U.S. to Triage and Expedite Incoming Referrals for the Evaluation of SMA
In July 2020, Cure SMA launched a survey to evaluate appointment wait time for initial referral, and current best practices to expedite and triage referrals across neurology and SMA care centers in the U.S. The initiative was designed to further understand referral patterns amongst providers, and identify potential contributors to existing diagnostic delays in SMA. The survey was distributed via Medscape to neurologists, child neurologists, and neuromuscular specialists (general cohort). Cure SMA also distributed the survey to providers affiliated with the Cure SMA Care Center Network to identify best practices utilized at these sites. The results revealed prioritized scheduling at Cure SMA Care Centers for patients suspected of having SMA due to symptomatic presentation (Figure 1), and infants diagnosed via newborn screening (Figure 2), to shorten wait times of initial appointments compared to the general cohort.
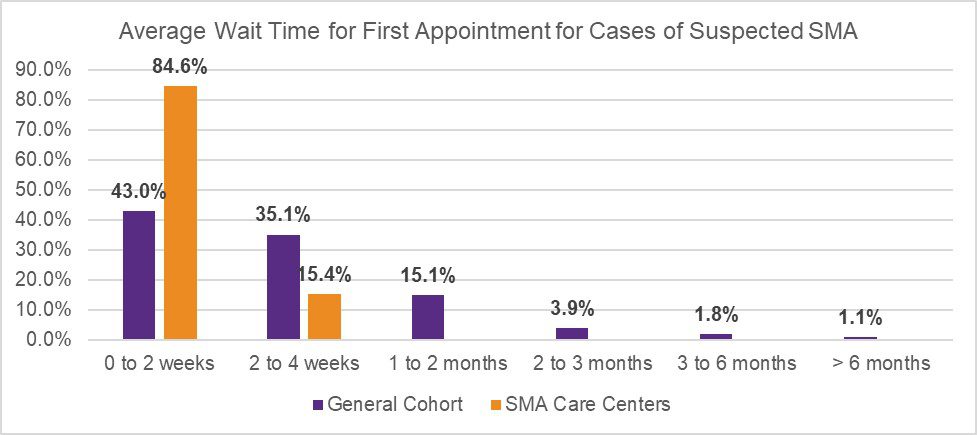
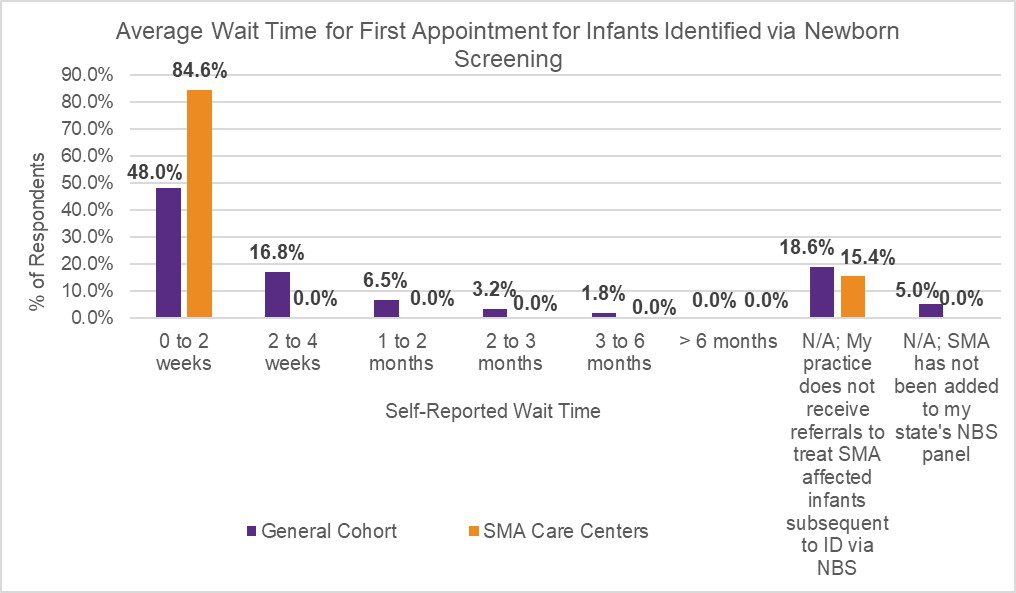
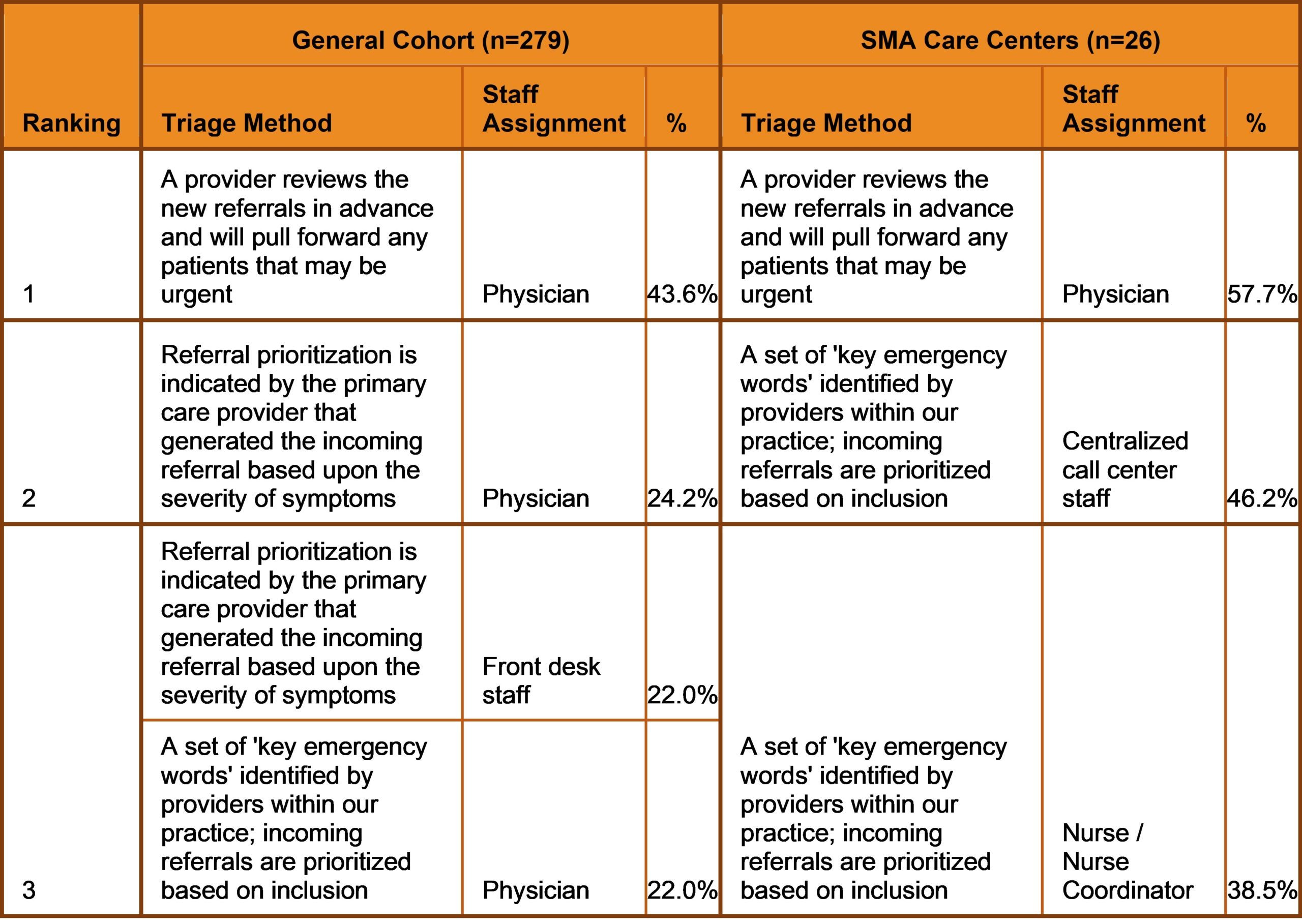
Additionally, although the majority of participants within the general cohort (84.6%) and SMA Care Centers (100.0%) triage incoming referrals to determine the priority of appointment scheduling at their sites, the results revealed a noted difference in triage methods utilized across both groups. While participants within the general cohort rely upon physicians to triage referrals, those linked to the Cure SMA Care Centers also utilize ‘Centralized Call Center Staff’ (46.2%) and ‘Nurse Coordinators’ (38.5%) to assist screening, and prioritize appointments if key emergency words are included within the referral (Table 1). The diagnosis of SMA is a medical emergency. Early diagnosis and treatment is critical to improving survival and modifying disease progression. The findings identify potential contributors to diagnostic delays across neurology and SMA care centers in the U.S.
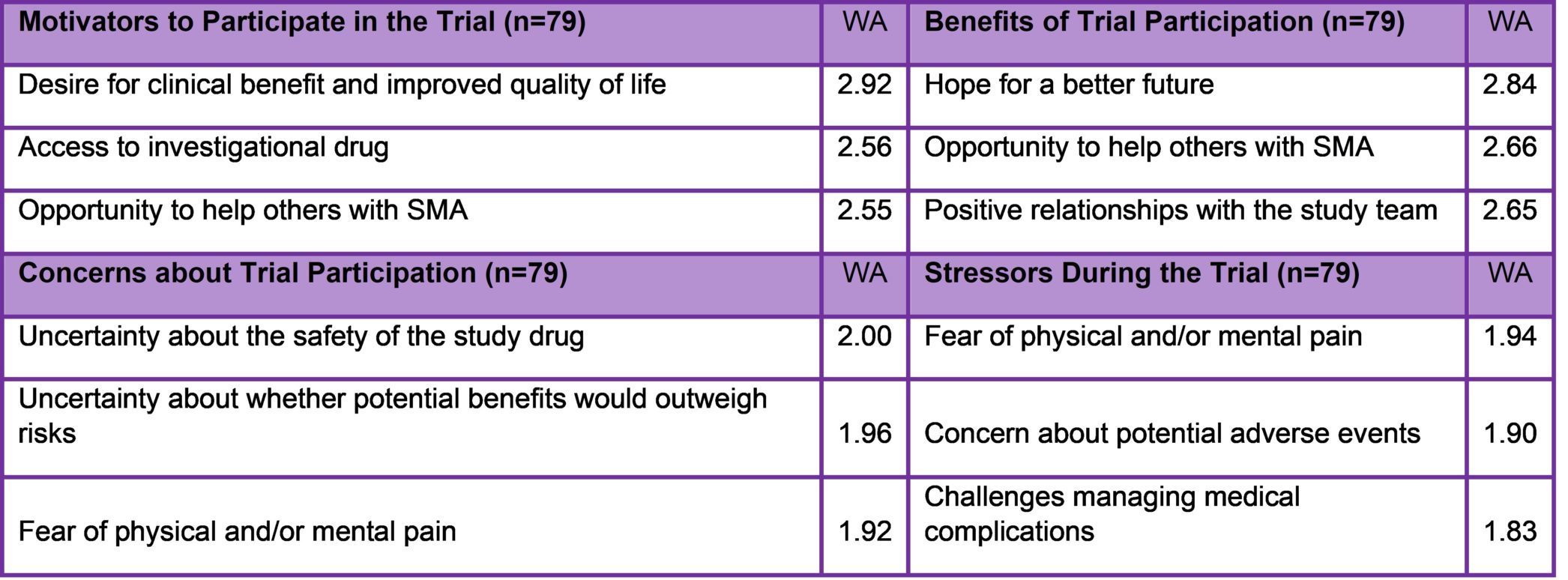
Results of 2019 Study on Clinical Trial Experiences of SMA Patients and Families
Participation in an SMA clinical trial can be an intensive experience. Cure SMA examined patient and caregiver experiences in SMA clinical trials to gain insight into motivations and expectations, and to identify opportunities for patient-centered management. The survey was fielded in late 2019 through early 2020. SMA trial participants and caregivers of majority age were eligible to complete the survey. Eighty-nine eligible individuals completed surveys reflecting 79 unique experiences. The top three motivators, concerns, stressors, and benefits associated with trial participation are presented in Table 2. The study also found that a few factors appears to help to reduce stress levels, including trust in the study team (weighted average of WA=2.73, where 3=significant), understanding expressed by the study team (WA=2.7), and witnessing improvements (WA=2.67). Results also indicated that higher levels of knowledge about SMA and clinical trials predicted lower stress levels. From these findings, the researchers concluded that patient-focused clinical trial management may improve trial participants’ experiences and sites/sponsors can pursue this by increasing access to information; building trusting relationships between study staff and participants; and proactively accommodating participant needs.
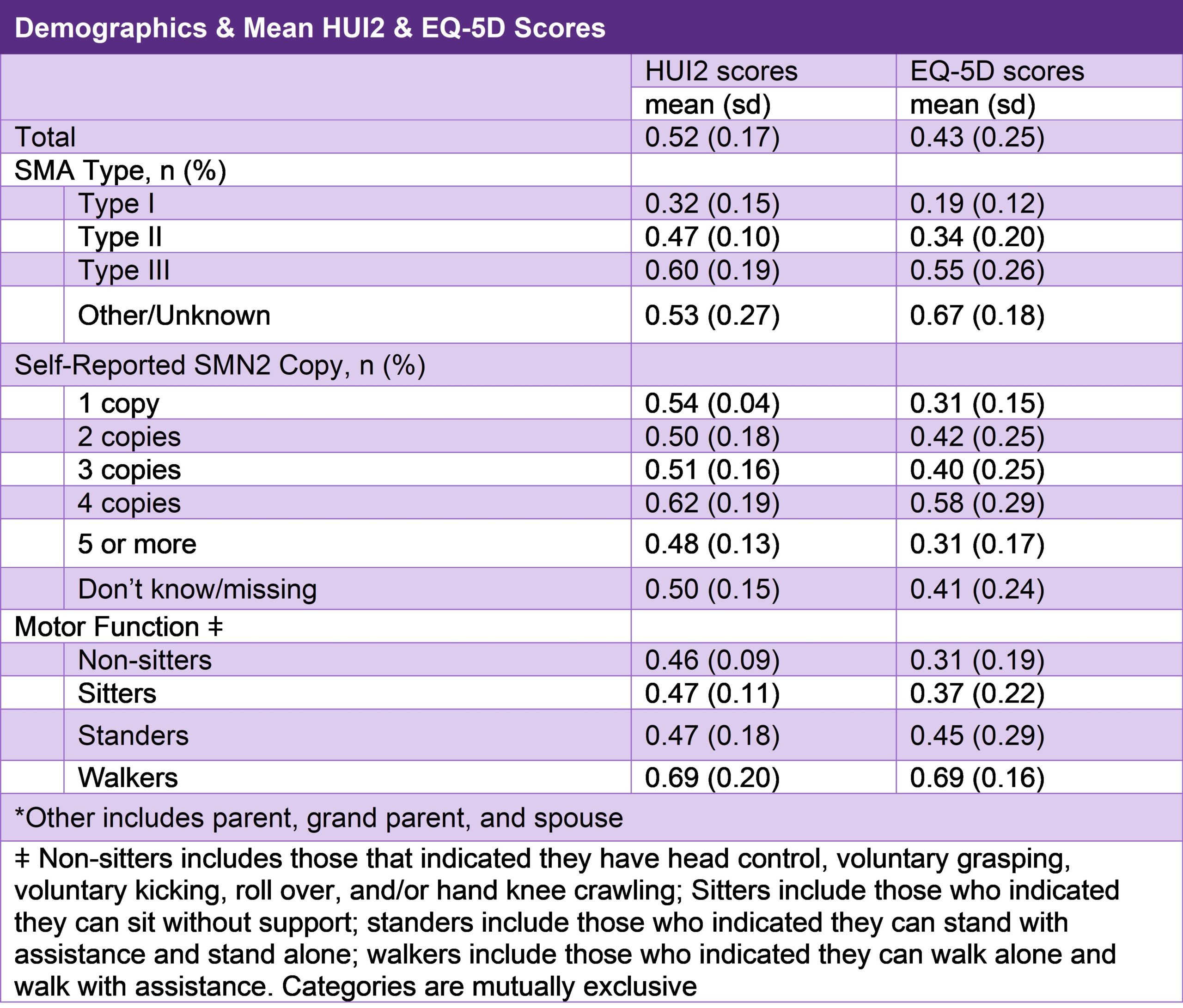
EQ-5D and HUI2 Results from the Cure SMA Annual Community Update Survey
In April 2020, Cure SMA launched its 4th Annual SMA Community Update Survey. One of the goals of the survey was to collect longitudinal data on the health status of individuals with SMA using the Health Utilities Index Mark 2 (HUI2) and the EQ-5D. The HUI2 can be used to derive health-related quality of life scores through the following 7 attributes: sensation, mobility, emotion, cognition, self-care, pain, and fertility, each with 3 to 5 levels. The final score can range from -0.36, representing a quality of life worse than death to 1.00, representing a quality of life equal to perfect health. The EQ-5D is a system that measures health-related quality of life through five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 3 levels: no problems, some problems, and extreme problems. The scores of each dimension can then be combined to a single value that range from 0.00, which represents a quality of life equal to death, to 1.00, which represents a quality of life of having perfect health.
The HUI2 was completed for 296 affected adults. The EQ-5D was completed for 339 affected adults. The results are shown in Table 3. Findings from this study highlight the severe burden experienced among the SMA community. The scores of both instruments are inversely correlated with disease severity, with the most affected scoring the lowest in health status. This study will allow us use these instruments in the future to measure changes in the quality of life in SMA patients over time.
Results from the Cure SMA SMN2 Copy and Motor Function Telephone Survey
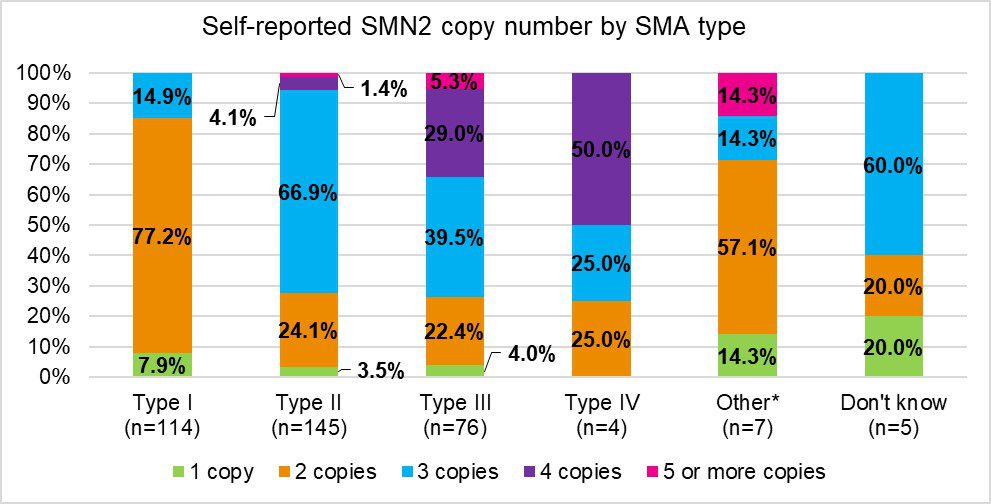
Cure SMA conducted a telephone survey with their database members in the summer of 2020 to collect missing self-reported information on SMN2 copy number and current motor function. These two outcomes are important to understand in the SMA community because the SMA phenotype has been evolving since regulatory approvals of SMA therapies. These therapies, coupled with improvements in supportive care, are leading to patients gaining motor functions that are atypical for their subtype. These changes in the SMA phenotype, as well as the increasing number of patients diagnosed before symptom onset due to statewide newborn screening, and relying on SMN2 copy number, which will not change over time regardless of treatment, is more useful than using SMA subtypes for describing SMA clinically. A total of 806 respondents completed the survey out of 2,807 contacts. The majority of respondents were caregivers and the majority of respondents completed a survey on behalf of someone with Type 2 SMA. We saw 43.6% of respondents reporting SMN2 copy numbers for the SMA patient. Figure 2 shows the self- or caregiver-reported SMN2 copy number by SMA type. Of the individuals that did not know the copy number, 14.6% stated it was because genetic testing was not done; however, other reasons for not knowing were not collected. Fortunately, due to the advent of FDA treatments for SMA, SMA type is becoming obsolete. The data from this survey will assist in future studies at Cure SMA in stratifying patients based on SMN2 copy number and current motor function due to the changing phenotypes of SMA in the era of newborn screening and effective disease modifying therapies.
About the Cure SMA Industry Collaboration
The Cure SMA Industry Collaboration (SMA-IC) was established in 2016 to leverage the experience, expertise, and resources of pharmaceutical and biotechnology companies, as well as other nonprofit organizations involved in the development of spinal muscular atrophy (SMA) therapeutics to address a range of scientific, clinical, and regulatory challenges more effectively. It is currently comprised of our partners at Novartis Gene Therapies, Biogen, Genentech/Roche Pharmaceuticals, Scholar Rock, and SMA Europe.
Funding for this research was provided by members of the 2019 and 2020 SMA-IC which included, Genentech/Roche, Novartis, Novartis Gene Therapies, Biogen, Cytokinetics, and Scholar Rock.


