Newborn Screening for SMA
Evidence shows that early diagnosis through newborn screening and early intervention with available treatments lead to better outcomes. This is especially true with spinal muscular atrophy (SMA), where early detection and timely administration of therapies can prevent the rapid and irreversible loss of motor function caused by the disease.
Quick Links
What Is Newborn Screening?
According to the Centers for Disease Control and Prevention (CDC), newborn screening identifies conditions that can affect a child’s long-term health or survival. Early detection, diagnosis, and intervention can prevent death or disability and enable children to reach their full potential. Each year, millions of babies in the U.S. are routinely screened—using a few drops of blood from the newborn’s heel—for certain genetic, endocrine, and metabolic disorders. They are also tested for hearing loss and critical congenital heart defects (CCHDs) prior to discharge from a hospital or birthing center.
In July 2018, the federal government added spinal muscular atrophy (SMA) to the Recommended Uniform Screening Panel (RUSP)—the list of suggested conditions that states should screen for within their statewide universal newborn screening programs. Since then, Cure SMA and its advocates have successfully encouraged the adoption of newborn SMA screening in all 50 states.
Frequently Asked Questions
SMA affects the nerves in the spinal cord that send signals to the muscles to tell them how to work. When these nerves don’t work, muscles can’t do their job and become very weak. People with SMA may have difficulty walking, eating, and breathing because of muscle weakness. The condition is serious and can lead to early death, but research shows that early diagnosis and early treatment can help.
When a baby is born, blood is taken to test for conditions that could affect the child’s health. If someone receives a positive result for SMA, the baby likely has SMA. The good news is that there are ways to treat SMA. A baby is tested at birth because it’s important to start treatment early. Though the baby may be healthy now, infants with SMA have a missing or faulty gene that can cause serious health problems later if not treated. A healthcare provider’s early determination about treatment offers the best chance that a baby will stay as healthy as possible.
The results of a newborn screening test show that a baby most likely has SMA. A doctor will order more tests to confirm the diagnosis. Additional tests may also provide information that helps predict how serious a case of SMA each child has.
Signs that a child has SMA (before receiving a treatment) will depend on the type of SMA the baby has. Historically, most cases of SMA are Type 1, which is the most serious. These symptoms appear within the first six months after birth. In some cases, like Types 2 and 3, symptoms may not appear until later. Depending on how serious a child’s condition is, the best chance to prevent dangerous muscle weakness in the child is to get treatment early. A child with SMA who is identified by newborn screening is often identified before signs and symptoms of SMA are present and are referred to as pre-symptomatic SMA.
SMA is caused by a missing or faulty gene, known as the SMN1 gene. Babies usually receive two copies of this gene—one from the mother and one from the father. A person with only one functioning SMN1 gene is deemed to be healthy, so parents may pass down a missing or faulty SMN1 copy without knowing it. A baby born with SMA has received a missing or faulty SMN1 gene copy from both parents.
A small number, approximately 5%, of children with SMA have a point mutation in the SMN1 gene that is not picked up by newborn screening or usual genetic testing for SMA. The 5% not identified by newborn screening will develop signs of SMA, and the diagnosis of SMA will require additional testing with SMN1 gene sequence analysis.
Currently, there are multiple FDA-approved treatments for SMA. Evrysdi® is a treatment developed by Genentech, a member of the Roche Group, and approved for the treatment of SMA in all ages and all types. Spinraza®, a treatment developed by Biogen and Ionis, is approved to treat all ages and types. Zolgensma® is a treatment developed by Novartis Gene Therapies to also treat all types of SMA in patients who are under two years of age. In addition to these approved treatments, several other treatments are being tested in clinical trials. If you are managing a new SMA diagnosis, make an appointment with your healthcare provider to discuss the best approach to treatment.
Take the following actions right away after receiving notice that your child has a positive newborn screen for SMA:
-
Contact the baby’s doctor or other healthcare provider and share these test results, if they have not been shared already
-
Ask the healthcare provider for a referral to a specialist, who will order more tests to confirm whether the baby has SMA. Often, though not always, the referral will be to a pediatric neurologist, a doctor who specializes in nerve diseases in children
-
Get more information to share with the baby’s healthcare provider and other caregivers. The more you know, the quicker decisions can be made
- Contact Cure SMA by emailing your mailing address to [email protected] so we can overnight you an information packet and provide you with support and resources
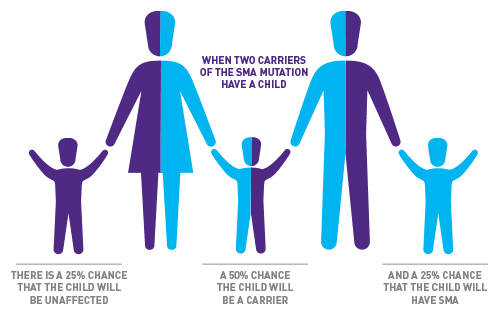
The figure above shows the chances that a healthy mother and father who are SMA carriers—each with one working SMN1 gene and one missing or faulty SMN1 gene—will have a child with SMA. In each pregnancy, the chance of these parents having a child with SMA is one in four, or 25%.
Newborn Screening Resources
We know this information may come as a surprise. We’re here to help. Cure SMA is nonprofit advocacy group that focuses specifically on SMA. You can find numerous resources for newly diagnosed families, and you can always contact us for information, guidance, or support. The below booklets offer a foundation for understanding SMA if you are a parent, patient, or healthcare provider learning about SMA for the first time.
Guide for Families: What You Need to Know and Do About an SMA Diagnosis
Guide for Healthcare Providers: What You Need to Know and Do About an SMA Diagnosis
To access and download all of our Cure SMA Care Series Booklets, click the button below
These booklets discuss topics from the genetics of SMA to nutrition, breathing basics, musculoskeletal system, mental health, and much more! Many of our booklets are offered in over ten languages.
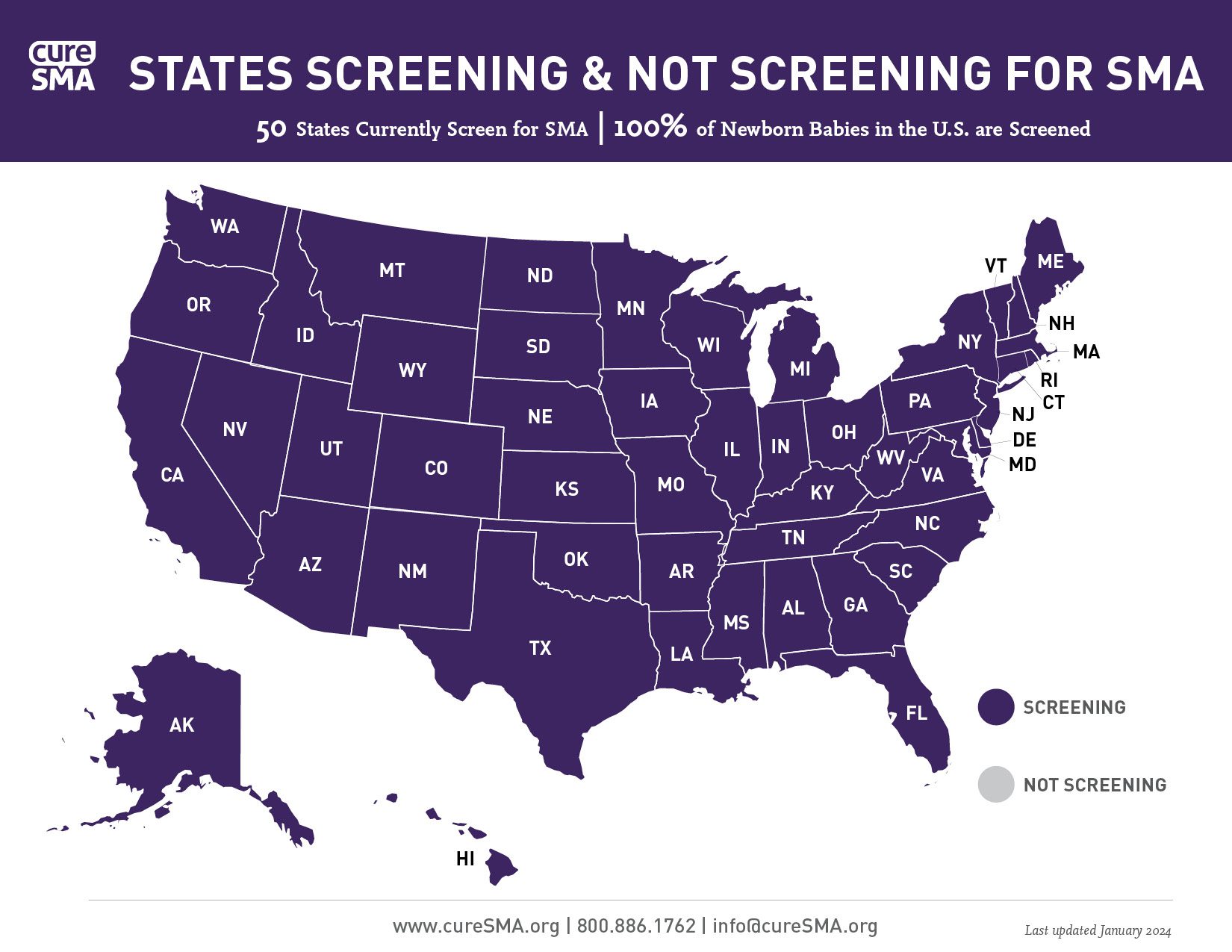
Is Your State Screening for SMA?
The status of newborn screening for SMA across the U.S. is shown on the following map — with all 50 states, plus Washington, D.C., screening for SMA. Within 6 years of SMA being added to the federally recommended list of diseases to screen for at birth, Cure SMA and its advocates have ensured that 100% of babies born in the U.S. are now screened for SMA at birth. To see our newborn screening state-specific fact sheets, please visit our Advocacy Overview page.
Download a PDF of the above newborn screening state program map, as of January 2, 2024.
The Cure SMA Newborn Screening Registry (NBSR)
The Newborn Screening Registry (NBSR) is an online registry established to help the SMA community (e.g., individuals with SMA, families, clinicians, and researchers) learn more about SMA, better manage symptoms over time, and develop new treatments.
The NBSR is a Cure SMA program. Cure SMA is the sole guardian of NBSR and its material. NBSR information can be used to improve clinical care and to support new therapy development. Registries in other diseases also have a long history of success in moving research and clinical care forward.
The NBSR is undergoing updates and will be available again later this year. Please check back. We look forward to your participation.