Winter 2018 Compass Now Available Online
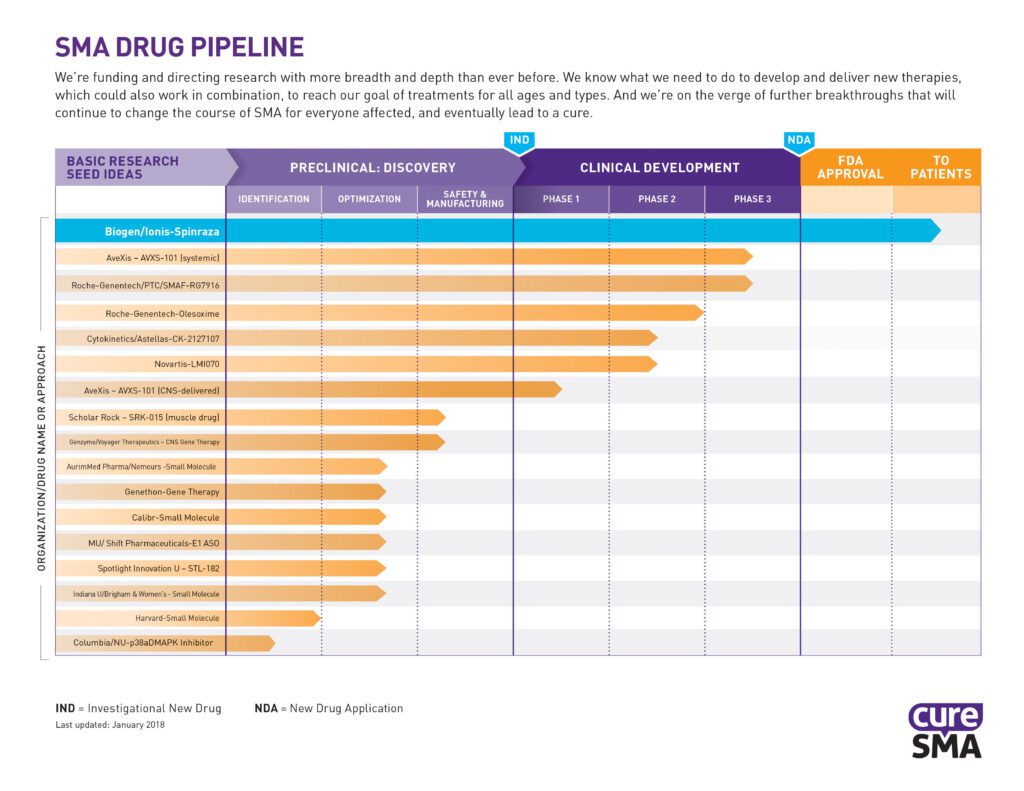
The winter 2018 issue of Compass is now available online. This issue covers Cure SMA’s community survey, and reviews how data is used to improve research, care and coverage in the SMA community. Cure SMA Community Update Survey Cure SMA has been collecting data through our database and various projects over the past several years. […]
Winter 2018 Compass Now Available Online Read More »