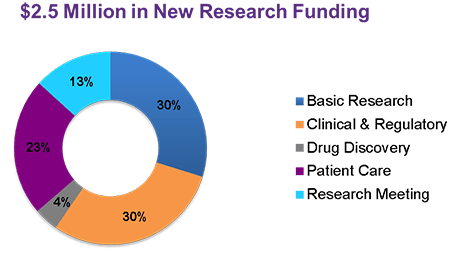
Cure SMA Announces $2.5 Million in New Planned Research Funding
At the 2016 Annual SMA Conference, Cure SMA announced $2.5 million in new planned research funding over the next 12 months. This funding will be used strategically to help accelerate research and ensure we are developing treatments for all types, ages, and stages of SMA. Funding Priorities As the SMA research landscape has developed and […]
Cure SMA Announces $2.5 Million in New Planned Research Funding Read More »