Approaches to Drug Development
Due to a mutation in the survival motor neuron gene 1 (SMN1), individuals with spinal muscular atrophy (SMA) don’t produce survival motor neuron (SMN) protein at high enough levels. Without this protein, the motor neuron cells shrink and eventually die, leading to the symptoms of SMA.
SMN-Dependent Treatments
One way to treat SMA is to increase the amount of SMN protein in the body. Treatments that increase the level of SMN protein are often called “SMN-dependent” approaches. These include:
Replacement or correction of the faulty SMN1 gene
The faulty SMN1 gene can be replaced or repaired through an approach called “gene therapy” or “gene replacement therapy.” Gene replacement therapy for SMA replaces the missing SMN1 gene.
A gene therapy called “Zolgensma®” has been approved by the U.S. Food and Drug Administration (FDA) for infants under two years of age with all types of SMA. Zolgensma® was developed and is marketed by Novartis Gene Therapies. It is a one-time intravenous (IV) infusion.
Modulation of SMN2, the low-functioning “back-up gene”
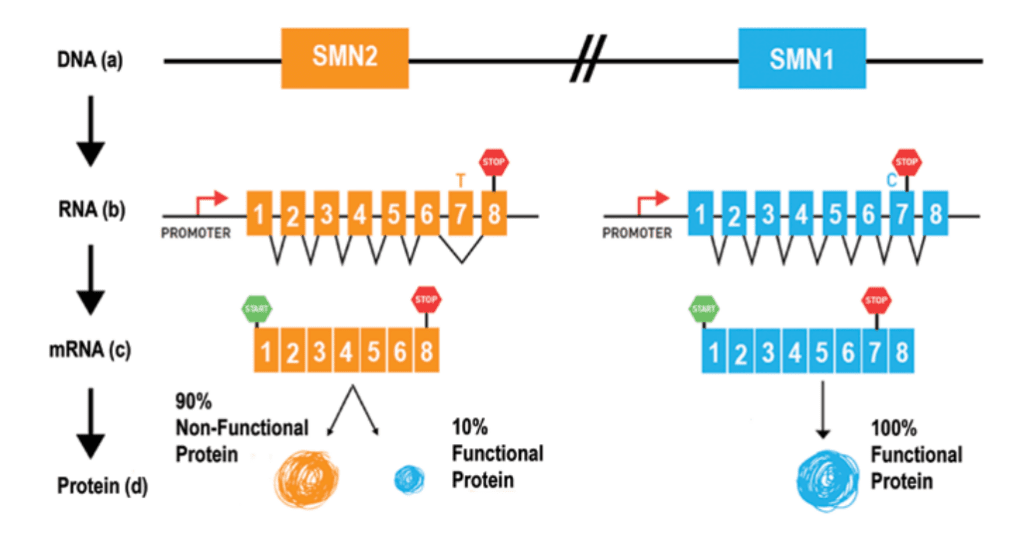
All individuals with SMA have at least one, and often multiple, copies of a second gene called “survival motor neuron gene 2 (SMN2).” SMN2 is sometimes referred to as the “SMN back-up gene.”
SMN2 also produces SMN protein, but only a small percentage of the protein produced by SMN2 is fully functional. (See figure above)
A number of strategies that target SMN2 are being explored:
- Correcting SMN2 mRNA splicing so that the SMN2 gene produces a complete protein
The FDA has approved two treatments that correct the way SMN2 is made into protein so that exon 7 is not missing.
Spinraza®, the first FDA-approved therapy for SMA, is a treatment that targets the SMN2 gene. Spinraza® is an antisense oligonucleotide (ASO) approved for all ages and types of SMA. Antisense drugs are small snippets of synthetic genetic material that bind to ribonucleic acid (RNA), so they can be used to fix the splicing of genes like SMN2.
Evrysdi®, an FDA-approved therapy for the treatment of SMA in all ages and all types, is another treatment that works by correcting the splicing of SMN2. Evrysdi® is a small molecule that is taken daily by mouth or by g-tube.
- Prompting SMN2 to make more protein
- Making the protein produced by SMN2 last longer
SMN-Independent Treatments
Increasing the amount of SMN protein in the body is not the only way to treat SMA. The loss of SMN protein also impacts other systems, pathways, and processes. Treatments aimed at these other targets are often called “SMN-independent” therapies. These approaches include:
Muscle protection to prevent or restore the loss of muscle function
When low levels of SMN protein disrupt the proper function of motor neurons, the loss of nerve stimulation causes the skeletal muscles to atrophy. The goal of muscle protection is to prevent muscles from atrophying, increase muscle mass, and perhaps even restore some muscle function.
While this strategy does not address the underlying genetics of SMA, it may help slow or stop the progression of SMA, and it can be used in combination with SMN-dependent approaches. SMN-independent approaches may include the use of small molecules that enhance the muscle’s ability to contract or regulators of muscle mass that may improve muscle strength.
Neuroprotection of the motor neurons affected by the loss of SMN protein
Motor neurons are one of the primary cell types affected in SMA. The goal of neuroprotection is to protect motor neurons by restoring their function and/or preventing their death. Researchers believe that neuroprotection could be used in combination with SMN-dependent therapies that fix the SMN1 or SMN2 gene. Two main neuroprotective strategies are being studied for SMA:
- Neuroprotective small molecules that help cells stay alive
- Stem cells transplants that provide growth factors to motor neurons
Newer approaches that identify additional systems and pathways affected by SMA
Many researchers believe that it will take a combination of SMN-dependent and SMN-independent treatments to provide the most benefit for those with SMA. This could mean that individuals with SMA will take two drugs together. Or, they may take one type of drug at one stage of the disease and then another drug at a different stage. For these reasons, SMN-independent treatments are sometimes referred to as “combination” or “add-on” therapies.
There are many SMN-dependent and SMN-independent therapies at various stages of development for SMA.